


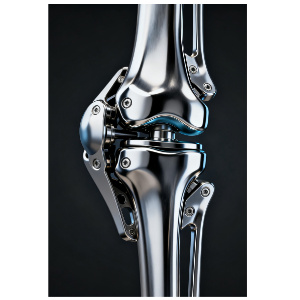
Artificial Joints
Covering hip joints and knee joints, they are made of titanium alloy and ceramic materials, and manufactured in accordance with Chinese national standards: GB/T 12417.1-2008 Implants for Surgery - Artificial Joints - Part 1: Hip Joint Prostheses and GB/T 41428.1-2022 Implants for Surgery - Artificial Knee Joints - Part 1: Knee Joint Prostheses. Meanwhile, they comply with international standards set by the International Organization for Standardization (ISO), including ISO 7206-1:2019 Implants for Surgery - Partial and Total Hip Joint Prostheses - Part 1: Requirements and Test Methods (for hip joints) and ISO 14879-1:2018 Implants for Surgery - Total Knee Joint Prostheses - Part 1: Requirements and Test Methods (for knee joints).
Titanium Alloy: Possesses excellent biocompatibility (meeting ISO 10993-1:2018 Biological Evaluation of Medical Devices - Part 1: Evaluation and Testing Within a Risk Management Process) and mechanical strength. It can withstand long-term activity loads, conforming to the mechanical property requirements for implant materials in ISO 5832-3:2019 Implants for Surgery - Metallic Materials - Part 3: Titanium-6 Aluminium-4 Vanadium Alloy.
Ceramic Materials: Feature low friction coefficient and high wear resistance, which can extend the service life of prostheses. They meet the specifications of ISO 6474-1:2018 Implants for Surgery - Ceramic Materials and Products - Part 1: Ceramic Materials for Hip Joint Prostheses.
These artificial joints are suitable for patients with severe joint diseases (such as rheumatoid arthritis, advanced osteoarthritis) or functional disorders caused by trauma. Through replacement surgery, they can relieve pain and restore the joint's flexion, extension, and weight-bearing functions.
Vascular Stents
Including coronary stents and cerebrovascular stents, they are made using nickel-titanium alloy weaving technology. They comply with Chinese standards: YY/T 0663.2-2024 Cardiovascular Implants - Vascular Stent Systems - Part 2: Coronary Stent Systems and relevant national/industry standards for cerebrovascular stents. Internationally, they align with ISO 25539-1:2021 Cardiovascular Implants - Vascular Stent Systems - Part 1: General Requirements and ISO 25539-3:2021 Cardiovascular Implants - Vascular Stent Systems - Part 3: Coronary Stent Systems.
Nickel-titanium alloy has shape memory properties and flexibility (meeting ISO 5832-11:2019 Implants for Surgery - Metallic Materials - Part 11: Wrought Nickel-Titanium Alloy), which can stably support the narrowed part of blood vessels and adapt to the anatomical curved shape of blood vessels.
Coronary Stents: Used to treat vascular stenosis/obstruction caused by coronary atherosclerotic heart disease, restoring myocardial blood supply. They meet the performance requirements for coronary stents in ISO 25539-3:2021, such as radial strength and fatigue resistance.
Cerebrovascular Stents: Targeted at cerebrovascular stenosis and cerebral aneurysms, preventing vascular rupture and bleeding, and ensuring cerebral blood circulation. They comply with the safety and effectiveness standards for cerebrovascular interventional devices in the Guidance on the Evaluation of Cerebrovascular Stent Systems issued by the International Medical Device Regulators Forum (IMDRF).
Compliance & Brand Assurance
All products have obtained approval from the National Medical Products Administration (NMPA) of China and are sourced from well-known brands such as Johnson & Johnson and Lepu Medical. These brands have mature R&D systems and strict quality control processes, in line with ISO 13485:2016 Medical Devices - Quality Management Systems - Requirements for Regulatory Purposes.
Product registration information and production processes can be traced through official platforms, ensuring that the entire process of R&D, production, circulation, and clinical use complies with national regulations, international standards (including ISO series standards and IMDRF guidelines), and prevents unqualified products from entering medical institutions.
Professional After-Sales Support
Professional personnel are arranged to assist medical institutions in checking the integrity of packaging, accuracy of quantity, appearance status, and standardization of labels (including registration code, batch number, production date, etc.) in accordance with national medical device inspection standards and product manuals. This process also references ISO 10360-12:2021 Geometrical Product Specifications (GPS) - Acceptance and Verification Tests for Coordinate Measuring Machines (CMM) - Part 12: Guidance for the Inspection of Medical Devices, ensuring the received products are qualified.
Surgical Use Training
Medical experts and technicians are invited to explain product characteristics, implantation operation steps, intraoperative precautions, and post-operative care points, combining national standard requirements and clinical cases. The training content aligns with the IMDRF Guidelines for Training in the Use of Medical Devices to help medical staff master the use methods proficiently and improve the success rate of surgeries.
24-Hour After-Sales Response
An all-weather team is established. When medical institutions encounter technical doubts, operation problems, or quality issues, they can contact the team at any time via phone, WeChat, or email. The team promises to respond within 24 hours and provide remote guidance or on-site services, ensuring the smooth development of diagnosis and treatment work. This service complies with the after-sales service requirements for medical devices in ISO 10002:2018 Quality Management - Customer Satisfaction - Guidelines for Complaints Handling in Organizations.
 Address:Room 1318, Unit A, Building 17, Headquarters Economic Park, No. 88 Gaohua Central Road, Tangquan Sub-district, Pukou District, Nanjing, Jiangsu, China
Address:Room 1318, Unit A, Building 17, Headquarters Economic Park, No. 88 Gaohua Central Road, Tangquan Sub-district, Pukou District, Nanjing, Jiangsu, China Tel:+86 18551848444 (Mr. Wang)
Tel:+86 18551848444 (Mr. Wang) E-mail:info@xyytrade.cn
E-mail:info@xyytrade.cn